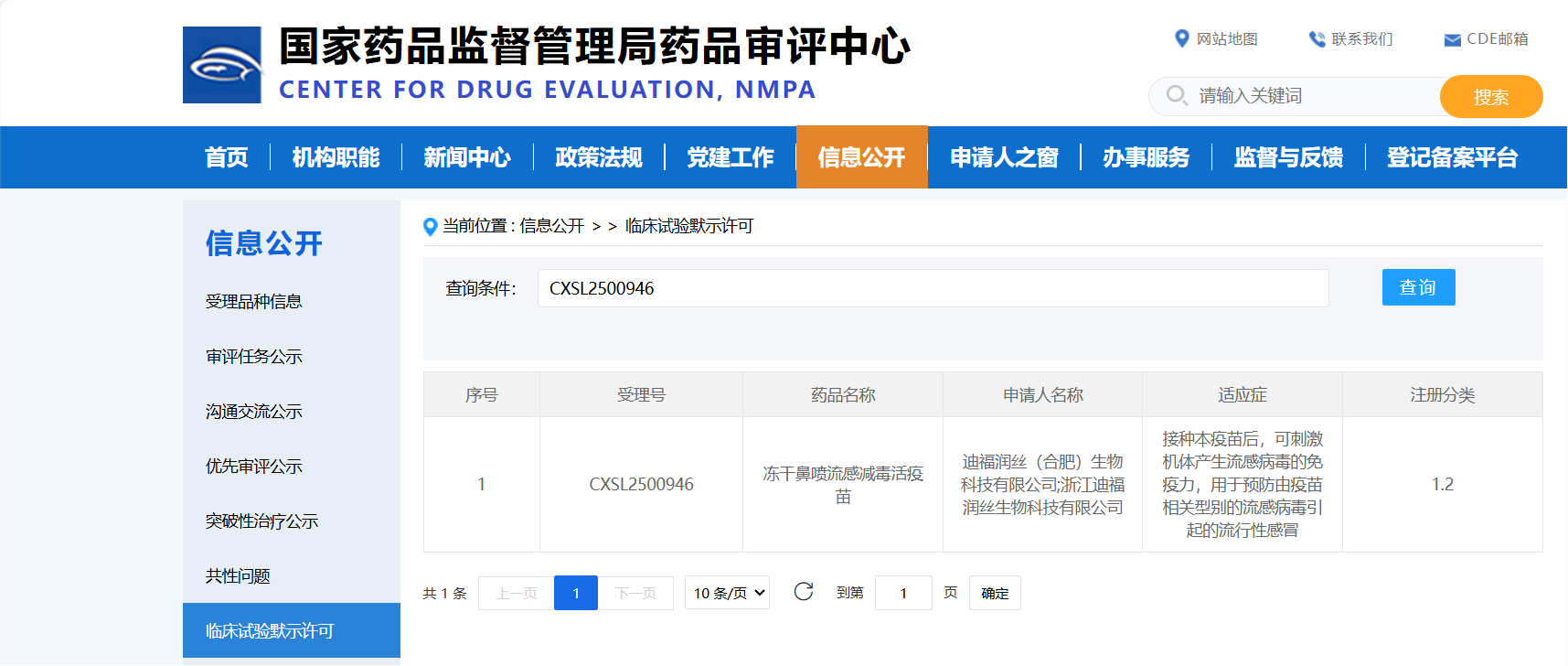
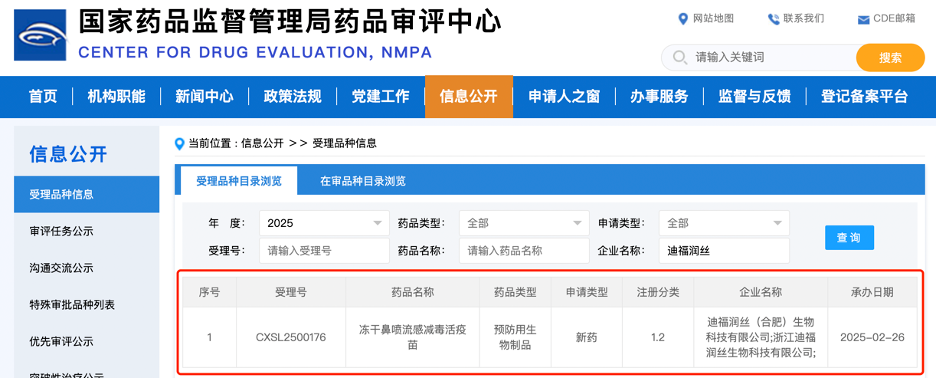
According to an announcement on the official website of the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) on February 26, the Investigational New Drug (IND) application for DIFF Biotech's "Lyophilized Nasal Spray Live Attenuated Influenza Vaccine" has been officially accepted (Acceptance No.: CXSL2500176). This vaccine is indicated for the prevention of influenza caused by relevant types of influenza viruses.
DIFF Biotech's lyophilized nasal spray live attenuated influenza vaccine is the first novel human nasal spray influenza vaccine in China with independent intellectual property rights. Utilizing the world's first M2 gene modification-based attenuation technology, the product has yielded a vaccine candidate strain with "restricted replication" capability, achieving more thorough attenuation and enhanced cross-protective efficacy against variant strains. It is expected to fill the technological gap in the field of influenza vaccine attenuation in China and has already obtained multiple domestic and international patent authorizations. According to preliminary non-clinical study results, this influenza vaccine demonstrates short or even no viral shedding after intranasal administration, low viral load in animal tissues, and minimal side effects, indicating higher safety and stability. Simultaneously, it can induce multi-tiered immune protection, including mucosal, humoral, and cellular immunity, covering different types/subtypes of Influenza A and B.