Global
We look forward to getting closer to you
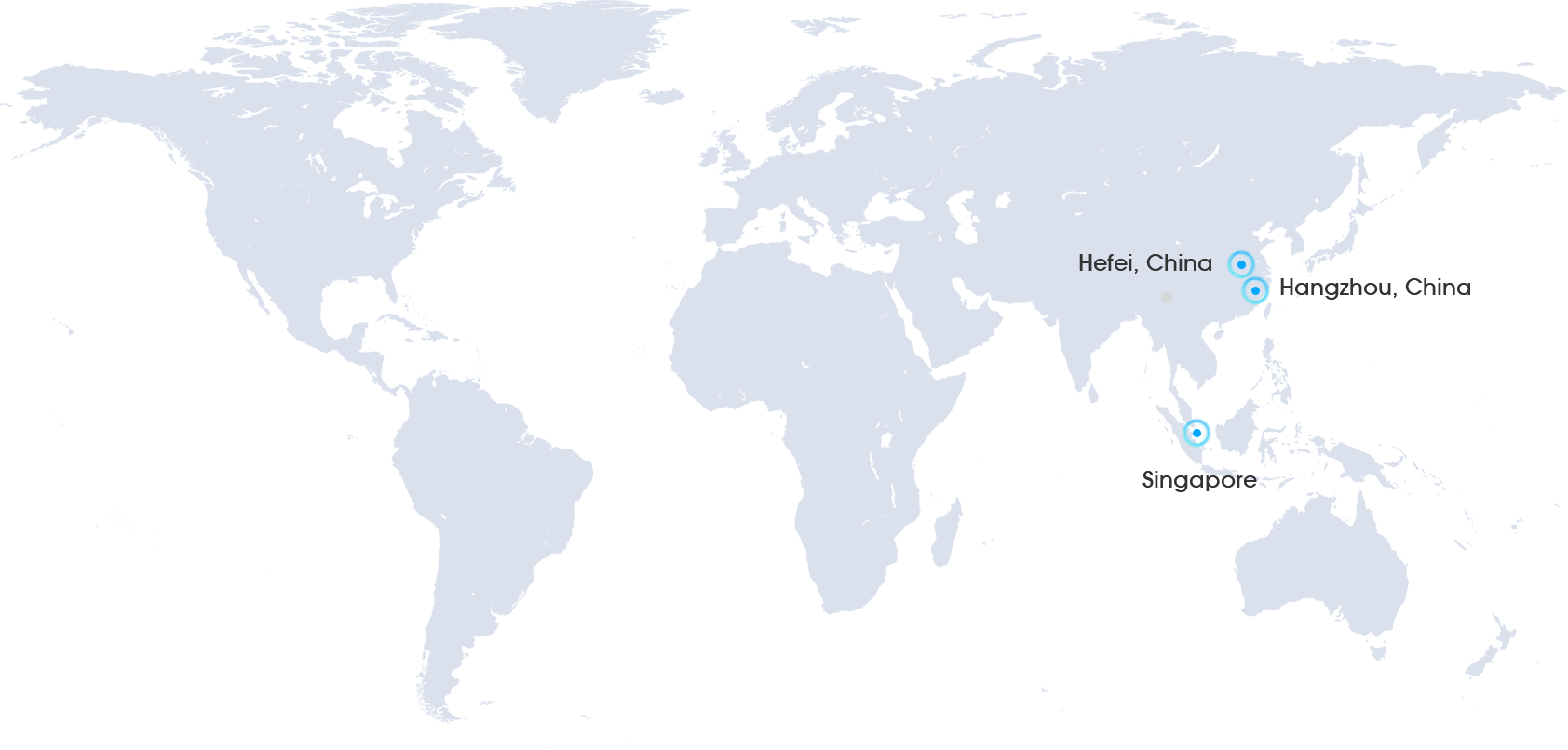
Currently, we are based on three global fulcrums and have deployed over 20 international patents in the United States, Europe, Brazil, South Africa, Thailand, Malaysia, and other countries along the Belt and Road.
Product
News